Download Free Report Sample At: https://www.vynzresearch.com/healthcare/obesity-surgery-devices-market/request-sample
Product recalls, the elevated price associated with surgeries, lack of appropriate healthcare infrastructure, limited insurance coverage and post-operative complications of bariatric surgery are some of the factors hindering the growth of the market.
The lack of awareness regarding diseases related to obesity, the selective nature of obesity surgery, and costly surgical procedure are the major challenges for the growth of obesity surgery devices market.
Emerging markets such as China and India and mounting medical tourism would create opportunities for the obesity surgery devices market.
Medical tourism is gaining traction with people touring abroad to seek improved quality and cost-effective medical, dental and surgical care. Presently, the expense is considered to be a significant factor when it comes to obesity surgery. Medical tourism for obesity surgery is mounting speedily with thousands of persons touring out of the U.S., South Africa, Canada, and Europe to undergo surgeries for this condition.
Asia-Pacific is observed to witness the fastest growth in the market, due to the escalating number of bariatric surgeries. In addition, escalating medical tourism, the mounting occurrence of the obese population, changing lifestyle, and government initiatives to limit obesity are also creating a positive impact on the obesity surgery devices market growth in the region.
Read More: https://www.vynzresearch.com/healthcare/obesity-surgery-devices-market
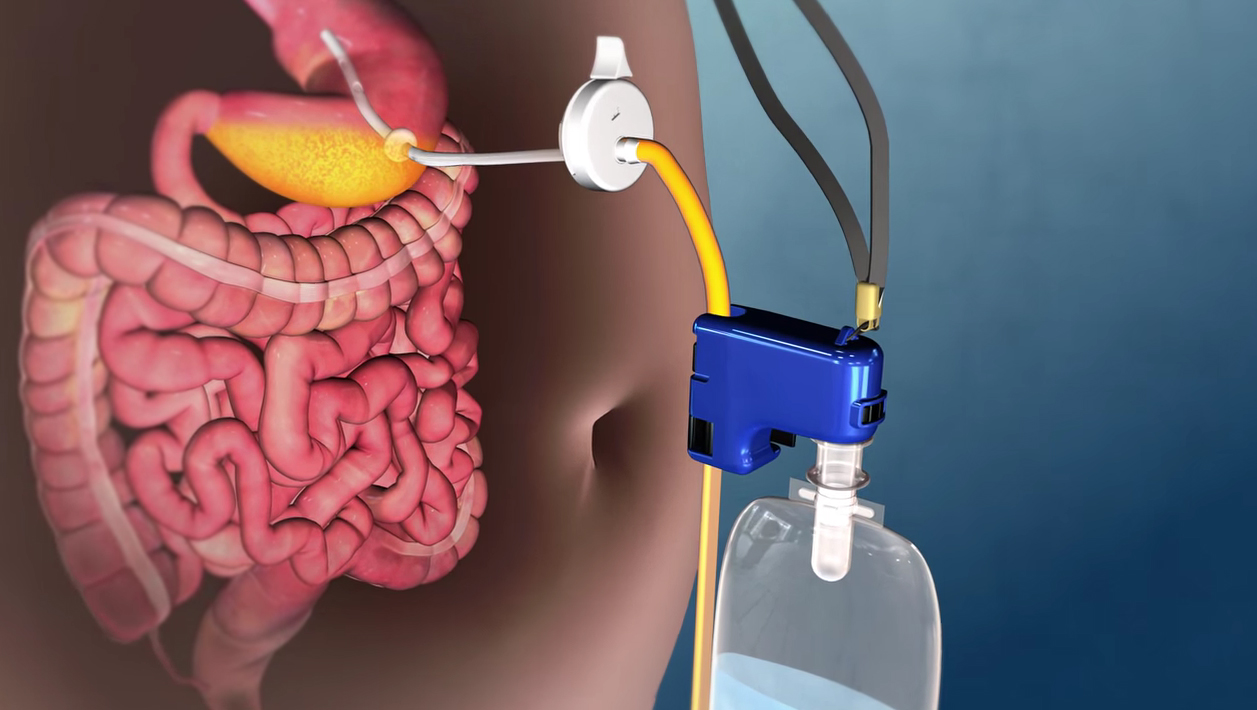
The obesity surgery devices industry players are catering to the demand of these devices by investing in technologically advanced products in their product portfolio across the globe. In June 2018, Apollo Endosurgery, Inc. received FDA approval of revised labeling for the ORBERA intragastric balloon system. Intuitive Surgical, Johnson & Johnson, Mediflex Surgical Products, Apollo Endosurgery, Medtronic Plc, Spatz Fgia, Olympus Corporation, Cousin Biotech, and Aspire Bariatrics are the key players offering obesity surgery devices.
About VynZ Research
VynZ Research is a global market research firm offering research, analytics, and consulting services on business strategies. We have a recognized trajectory record and our research database is used by many renowned companies and institutions in the world to strategize and revolutionize business opportunities. The company focuses on providing valuable insights on various technology verticals such as Chemicals, Automotive, Transportation, Energy, Consumer Durables, Healthcare, ICT and other emerging technologies.
Contact
Manager: Client Care
Toll-Free: 18882533960
Email: kundan@vynzresearch.com
Website: www.vynzresearch.com
No comments:
Post a Comment